Abstract
Background: Obinutuzumab (G) is an anti-CD20 monoclonal antibody approved in the US for the first-line (1L) treatment of follicular lymphoma (FL), relapsed or refractory FL, and 1L chronic lymphocytic leukemia. G plus chemotherapy (G-chemo) demonstrated superior progression-free survival versus (vs) rituximab (R) plus chemotherapy (R-chemo) in patients with previously untreated FL in the Phase III, randomized GALLIUM study (NCT01332968; Marcus et al. N Engl J Med 2017). R-bendamustine (R-benda) and G-bendamustine (G-benda) are among the most commonly used chemoimmunotherapy (CIT) regimens for FL (Ta et al. J Clin Oncol 2021), and information on comparative healthcare resource use (HRU) and real-world costs associated with G-chemo vs R-chemo in previously untreated FL patients is limited. The aim of this study was to compare HRU and costs for G-benda and R-benda for the 1L treatment of FL using US claims databases.
Methods: This was a retrospective cohort study using administrative claims data from the IQVIA PharMetrics® Plus and IBM® MarketScan Commercial and Medicare Supplemental databases. We identified patients aged ≥18 years, who had ≥1 inpatient claim or ≥2 outpatient claims with a diagnosis of FL from February 1, 2015 to September 30, 2020, and received 1L R-benda or G-benda between February 1, 2016 and March 31, 2020. The first claim for FL treatment was the index date. All patients had ≥12 months of pre- and ≥6 months of post-index continuous enrollment in medical and pharmacy benefits. Patients with other primary cancers, FL treatment, diffuse large B-cell lymphoma (DLBCL), or stem cell transplant during the pre-index period, or clinical trial participation or end-stage renal disease during the study period were excluded. Patients initiating 1L G-benda were propensity score matched 1:2 with patients initiating 1L R-benda based on age, sex, Charlson Comorbidity Index (CCI), region, and insurance type. All-cause and FL-related (i.e. claim with a FL diagnosis in any position) HRU and costs (2020 USD) per patient per month (PPPM) during the follow-up period were reported. Patients were followed until the earliest of initiation of second-line therapy, end of continuous follow-up, or end of data availability.
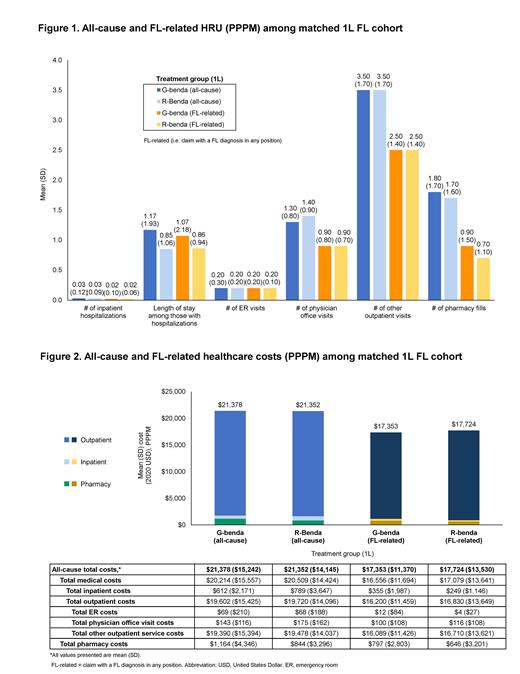
Results: Overall, 270 patients were included; of these, 90 (33.3%) patients receiving G-benda were matched to 180 (66.7%) patients receiving R-benda for 1L treatment of FL. 45.2% were male, and the mean (standard deviation [SD]) age and CCI at index date were 59.1 (9.9) years and 1.7 (1.1), respectively. Median follow-up was 7.4 months. After matching, baseline characteristics were well-balanced between R-benda and G-benda patients (standardized mean difference [SMD] <0.1). Both all-cause and FL-related HRU were similar between R-benda and G-benda treated patients across all service categories (Figure 1). Total all-cause mean [SD] PPPM costs were also similar in G-benda vs R-benda patients ($21,378 [$15,242] vs $21,352 [$14,145]; p=0.77) (Figure 2). The majority of the total costs were FL-related and comparable across both patient groups: $17,353 ($11,370) for G-benda vs $17,724 ($13,530) for R-benda (p=0.71). Specifically, FL-treatment related costs PPPM were $17,070 ($14,064) for G-benda; this is comparable to $16,138 ($11,828) PPPM for R-benda (p=0.39).
Conclusions: Our study found similar total costs of care and HRU among patients receiving 1L G-benda versus those receiving R-benda, providing real-world economic evidence that complements clinical trial data supporting the use of G-chemo for 1L treatment of FL.
To: Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Ta: Genentech, Inc.: Current Employment. Seetasith: Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company. Wang: The SPHERE Institute: Ended employment in the past 24 months; Aurinia Pharmaceuticals Inc.: Current equity holder in publicly-traded company; Novavax, Inc.: Current equity holder in publicly-traded company; Oragenics, Inc.: Current equity holder in publicly-traded company; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment; TG Therapeutics, Inc.: Current equity holder in publicly-traded company. Lai: Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company; AbbVie: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months; Bristol-Myers Squibb: Current equity holder in publicly-traded company. Shapouri: Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal